To measure the density of a sample of material, both the mass and volume of the sample must be determined. For both solids and liquids, a balance can be used to measure mass; however, methods for determining volume are different for solids and liquids. As liquids can flow and take the shapes of their containers, glassware such as a graduated cylinder or volumetric flask can be used to measure the volume of a liquid.
The volume of an irregularly-shaped solid can be measured by submersion in a liquid — the difference in volume caused by addition of the solid is equal to the volume of the solid. Most solid substances are irregularly shaped, which complicates volume determination. It is inaccurate, for example, to determine the volume of a powder by measuring its dimensions.
A graduated cylinder containing a known volume of liquid is tared. The solid is added to the cylinder, and the total mass is weighed again to determine the mass of the solid. Addition of the solid causes an upward displacement of the liquid, resulting in a new volume reading. The volume of the solid is equal to the change in volume due to liquid displacement (i.e., the difference in liquid volume before and after adding solid). Note that pure water (as opposed to sea water, which is 3% denser) has a density of 1,000 kilograms per cubic meter, or 1 gram per cubic centimeter.
To determine the density of an irregular solid in pellet form, add approximately 40 mL of water to a clean and dry 100-mL graduated cylinder. Place the cylinder on an analytical balance and tare. Add approximately 10 pellets, and record the new volume after the addition. The mass is only the pellets, as the rest have been tared.
Make at least two additional sets of mass and volume measurements to calculate an average value of the density. The density for zinc was measured for three different samples. Note that, since the measurements were made in a graduated cylinder, which is less precise than a volumetric flask, the density has lower degree of precision. Except for objects with simple shapes, it is difficult to determine the volume directly.
Water is the most convenient liquid to use, but if an object cannot be immersed in water, then organic solvents such as ethanol or acetone can be used. The density of the object can be calculated from the two weight measurements and the density of the liquid. Of a substance is the ratio of the mass of a sample of the substance to its volume.
The SI unit for density is the kilogram per cubic meter (kg/m3). Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 . Table \(\PageIndex\) shows the densities of some common substances. Relative density, or specific gravity, is the ratio of the density of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water at its densest (at 4 °C or 39.2 °F); for gases, the reference is air at room temperature (20 °C or 68 °F).
The term "relative density" is often preferred in scientific usage. Volume is an amount of space, in three dimensions, that a sample of matter occupies. The number and the phase of the molecules in the sample primarily determine the volume of a substance. Volume will be measured in many ways in this course, but the units are usually milliliters or cubic centimeters .
Methods for determining or delivering precise volumes include volumetric pipets and pycnometers; less precise methods include burets, graduated cylinders, and graduated pipets. Table 1 lists results for the determination of the density of ethanol using a 50-mL volumetric flask. Densities were calculated by dividing the measured mass by 50.0 mL. Table 2 lists results for the determination of the density of a sample of zinc metal using a 100-mL graduated cylinder and the liquid displacement method.
Note that the measured densities are constant for both substances. Table 2, in particular, demonstrates that density is independent of the amount of substance studied. Generally, the density of water (which is approximately about 1 gram/cubic centimeter) is taken as the standard value for calculating the density of substances. However, the SI unit of Density is measured using kilograms per cubic meter (kg/m3).
Relative density can be calculated directly by measuring the density of a sample and dividing it by the density of the reference substance. The density of the sample is simply its mass divided by its volume. Although mass is easy to measure, the volume of an irregularly shaped sample can be more difficult to ascertain. One method is to put the sample in a water-filled graduated cylinder and read off how much water it displaces. Alternatively the container can be filled to the brim, the sample immersed, and the volume of overflow measured. The surface tension of the water may keep a significant amount of water from overflowing, which is especially problematic for small samples.
For this reason it is desirable to use a water container with as small a mouth as possible. This demonstration illustrates the methods for measuring the density of solids and liquids. Using a volumetric flask and an analytical balance, the density of ethanol can be determined. Using a graduated cylinder, analytical balance, and water as the displaced liquid, the density of zinc metal can be determined. Volume is the amount of space an object occupies while density is the mass of an object per unit volume.
You need to know the volume of an object before you can calculate its density. Calculating volume for regular objects can be done with a simple formula determined by the shape of the object. Common units for volume are cubic centimeters , cubic meters , cubic inches , and cubic feet . Once you have the volume, density is one more simple calculation away.
Common units for density are grams per cubic centimeter (g/cm3) or grams per milliliter (g/mL). Specific gravity is the ratio between the densities of two objects or substances, and it is expressed as a number without units of measure. Due to the value of 1 g/cm 3 for water, it is easy to determine the specific gravity of a given substance, which will have the same number value as its density. For example, the specific gravity of concrete, which has a density of 2.3 g/cm 3 , is 2.3. The specific gravities of gases are usually determined in comparison to the specific gravity of dry air.
Most rocks near the surface of Earth have a specific gravity of somewhere between 2 and 3, while the specific gravity of the planet itself is about 5. How do scientists know that the density of Earth is around 5 g/cm 3 ? The computation is fairly simple, given the fact that the mass and volume of the planet are known. In the same way, calculations regarding the density of other objects in the Solar System provide a clue as to their interior composition. The density of material shows the denseness of that material in a specific given area. A material's density is defined as its mass per unit volume.
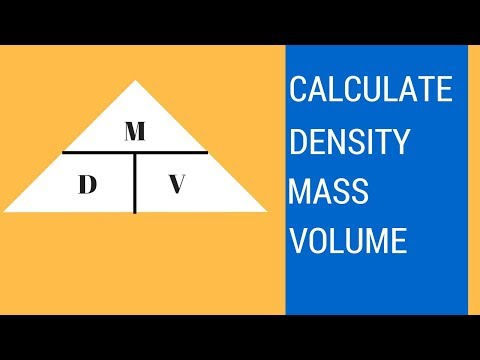
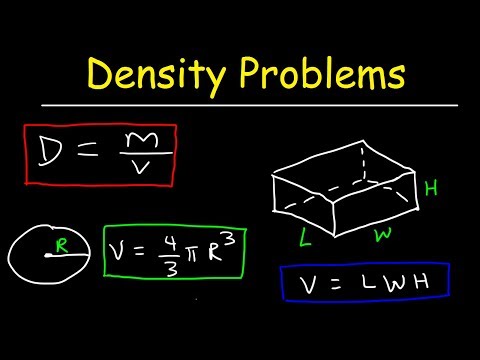
Density is essentially a measurement of how tightly matter is packed together. It is a unique physical property for a particular object. The principle of density was discovered by the Greek scientist Archimedes. It is easy to calculate density if you know the formula and understand the related units The symbol ρ represents density or it can also be represented by the letter D. Density can be determined by measuring the mass and volume of an object.
In the Density Bags Activity, density was not calculated. Instead, relative density was determined by observing whether a bag of one liquid floated or sank in another liquid. A bag of liquid that sank was determined to be more dense than the liquid in the beaker. A bag of liquid that floated was determined to be less dense than the liquid in the beaker. However, the density of a solid material can be defined in several ways.
Porous or granular materials have a density of the solid material, as well as a bulk density, which can be variable. For example, if you gently fill a container with sand, and divide the mass of sand by the container volume you get a value termed loose bulk density. Tapped bulk density is always greater than or equal to loose bulk density. In both types of bulk density, some of the volume is taken up by the spaces between the grains of sand. The density of the sand grains, exclusive of the air between the grains, will be higher than the bulk density.
If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density less than 1 will float in water.
For example, an ice cube, with a relative density of about 0.91, will float. A substance with a relative density greater than 1 will sink. Calculate the uncertainty in the mass of water removed using error propagation.
Convert this mass to volume units by dividing by the density of water (use a precise value, specific to the water's temperature). This value equals the uncertainity in the volume of the metal cylinder. Students use the water displacement method to find the volume of different rods that all have the same mass.
They calculate the density of each rod, and use the characteristic density of each material to identify all five rods. Then students consider the relationship between the mass, size, and arrangement of atoms to explain why different rods have different densities. Students will be briefly introduced to the periodic table. True density is the ratio between the mass and volume of a substance at a given pressure and temperature, corresponding to its weight in a vacuum. This is the concept also used in density measurement by digital density meters.
A simpler but less accurate way to measure the density is to place the object in a liquid and measure the volume of liquid displaced. This can be used on small objects that fit into a graduated cylinder, for example, to decide if the object is made of lead or a less dense metal. The volume can be measured by approximating the dimensions of an object, but a more reliable method is by using water displacement. Measure the volume of water poured into a graduated cylinder, then place the object in the water and remeasure the volume.
The difference between the two volume measurements is the volume of the object. Then a container filled to the brim with water is weighed, and weighed again with the sample immersed, after the displaced water has overflowed and been removed. Subtracting the last reading from the sum of the first two readings gives the weight of the displaced water. The relative density result is the dry sample weight divided by that of the displaced water. This method allows the use of scales which cannot handle a suspended sample. A sample less dense than water can also be handled, but it has to be held down, and the error introduced by the fixing material must be considered.
To use the water displacement method, an object is inserted into a graduated cylinder partially filled with water. The object's volume occupies space, displacing liquid and raising the water level. The difference between the two volumes, before and after the object was inserted, is the object's volume. Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3. However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects.
Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3). Now that you have determined the shape, which formula to use, and made the necessary measurements, you can calculate volume. By plugging in the values of your measurements and doing the math. Your finished product is the volume of your object.Remember to express your answer in cubic units. Whether you are using metric or SI, the unit of volume will always be cubic.
Be sure to always add units to the end of your calculation. Let's discuss the "common" units of density, and the "official" units. The most common unit for density is grams per cubic centimeter, or g/cm3.
For example, the density of water is one gram per cubic centimeter, and the density of lead is 3.42 g/cm3. The official units for density, known as SI units are kilogram per cubic meter (kg/cm3). Other equivalents are grams per milliliter g/mL and kilograms per liter, kg/L.
If the flask is weighed empty, full of water, and full of a liquid whose relative density is desired, the relative density of the liquid can easily be calculated. The particle density of a powder, to which the usual method of weighing cannot be applied, can also be determined with a pycnometer. The powder is added to the pycnometer, which is then weighed, giving the weight of the powder sample.
The pycnometer is then filled with a liquid of known density, in which the powder is completely insoluble. The weight of the displaced liquid can then be determined, and hence the relative density of the powder. It's used to measure the ratio of the density of a liquid grape to that of pure water. A hydrometer is basically a sealed tube which is narrow at the top and is weighted with a dense material such as lead at the bottom.

























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.